MicroSphere Technology: MSR™ – MicroSphere Refiner
Microsphere Drug Production: Redefined
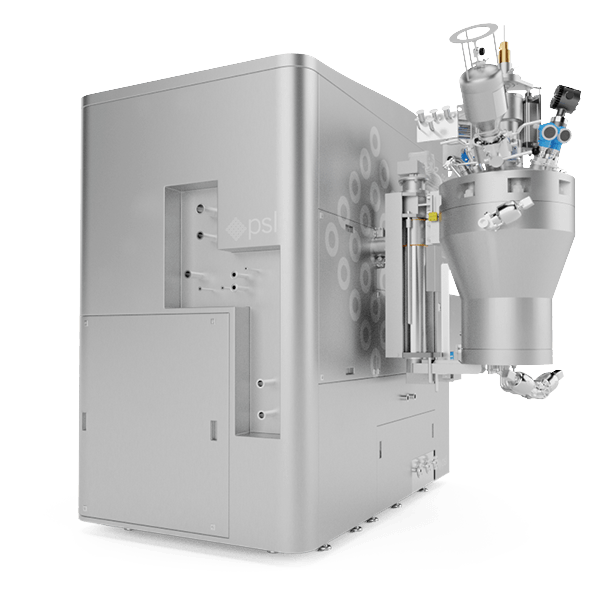
The MSR™ MicroSphere Refiner is a cGMP piece of equipment that allows drug developers and manufacturers to complete a wide range of aseptic processes with their microspheres at various scales. It is a one-of-its-kind solution has been developed and refined over decades following a Quality-by-Design (QbD) approach, taking into account the characteristics of microspheres and process behaviour.
Due to their characteristics and properties, polymeric microspheres have traditionally been known to be difficult to process for manufacturers – especially as the batch size gradually increases as part of the microsphere product development. The unique features of the MSR™ MicroSphere Refiner were pioneered by analysing these systemic industry challenges and developing a better understanding of microsphere processes with end-users directly in the field.
Features and Benefits
An All-In-One Solution
The MSR™ MicroSphere Refiner is a Current Good Manufacturing Practice (cGMP) piece of equipment that allows drug manufacturers to complete a wide range of aseptic processes with their microspheres at various scales. It naturally maximises product recovery, batch repeatability and production flexibility as it is capable of completing the following processes in one single system:
Batch Reproducibility – At The Touch Of A Button
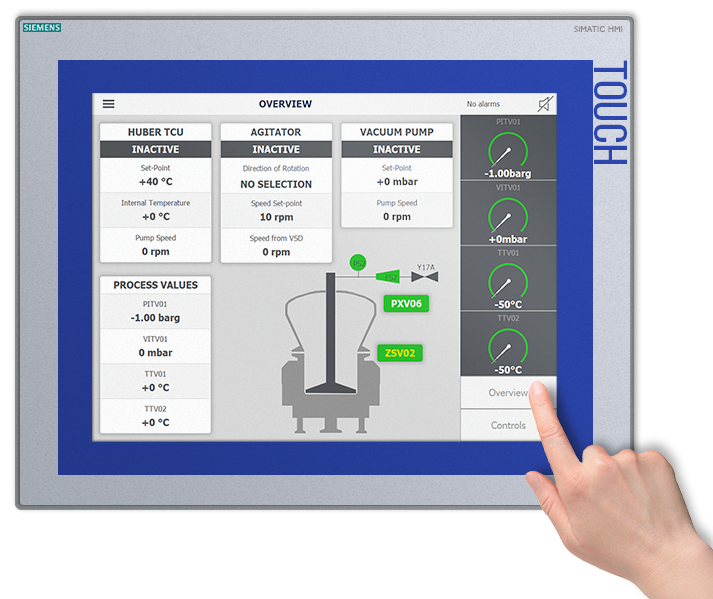
The MSR™ MicroSphere Refiner is a fully automated system designed to remove manual product handling by operators throughout production. This enables maximising batch-after-batch reproducibility while minimising the risk for human error. Production can start at the touch of a button and pre-designed process recipes can be initiated.
The PSL automation platform allows drug manufacturers to pre-design and pre-load software process recipes incorporating the relevant process parameters suitable for their specific production requirements. This adds speed and flexibility to their manufacturing processes.
PSL can comply to major international practices and regulations for software automation, including GAMP5 (Good Automated Manufacturing Practice, version 5 by ISPE) and 21 CFR Part 11 established by the US FDA for electronic records and signatures.
Product Recovery – A Crucial Requirement
As Microspheres are extremely valuable medicines to develop and produce it is vital that drug manufacturers maximise their product recovery in order to secure their competitive position in the global market. The core features of our MSR™ system were designed with this crucial requirement in mind.
The product recovery method of our MSR™ MicroSphere Refiner is unique as it is fully automated and it does not require any manual product off-loading by operators through an aseptic isolator, for example. Being an all-in-one solution, the MSR™ method also minimises the number of transfers and connections to aid maximising product yield.
Production Agility, Delivered
The MSR™ is a Good Manufacturing Practice (GMP) piece of equipment suited to multi-product applications. It is designed to allow end-users to remain agile and quickly adapt to changing production requirements.
| FILTRATION AGILITY | DRYING AGILITY | CLEANING AGILITY |
|---|---|---|
| One modular vessel design | Positive temperature drying | GMP design / no product traps |
| Quick filtration media change-over | Freeze-drying | CIP spray-ring option |
| Multi-zone filtration | Multi-zone drying | Suitable for potent product processing |
Your Process, PAT-Verified
The implementation of Process Analytical Technology (PAT) in the pharmaceutical industry is encouraged by the US FDA to ensure better product quality control and quicker time to market.
The MSR™ technology has embraced the PAT initiative as it allows drug manufacturers to complete real-time monitoring of their microsphere product quality and integrity during various production stages such as filtration and drying processes (including lyophilisation).
The integration of PAT reduces a trial and error approach. This helps drug companies move towards right first time manufacturing and reduces drug development times for microspheres – traditionally more demanding than regular drugs.

Successful Scale-up
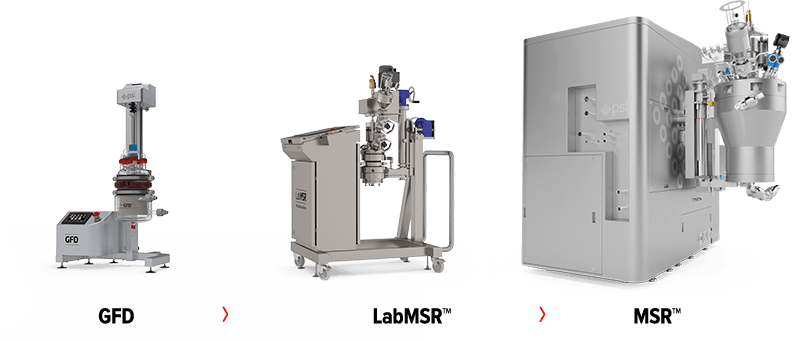
Developing complex drug formulations such as microsphere delivery systems has traditionally been challenging for most drug manufacturers, big or small. Ensuring that the chosen process technology used at R&D stage can be fully scalable to larger production batches is crucial. Finding out too late that a microsphere process is not scalable can have devastating impact and cost millions of dollars to drug manufacturers.
The MSR™ product range was designed to simplify the scale-up development of microsphere drugs, from early R&D activities to GLP batches and then from clinical trials to commercial production. Our solutions can ensure that key process objectives (such as product yield, product quality and integrity, batch consistency, etc.) and key production parameters (such as filtration time, washing time, drying time, etc.) can be maintained at every scale.