Predictive Microsphere Scale-up
The LabMSR™ system is a GLP piece of equipment that has been developed to enable global drug manufacturers to generally replicate, at R&D scale, some of the process benefits of PSL’s full scale production MicroSphere Refiners (MSR™).
Due to their characteristics and properties, polymeric microspheres have traditionally been known to be difficult to process for manufacturers – especially as the batch size gradually increases as part of the microsphere product development. The unique features of our LabMSR™ were developed following a Quality-by-Design (QbD) approach, taking into account the microspheres characteristics and process behaviour.
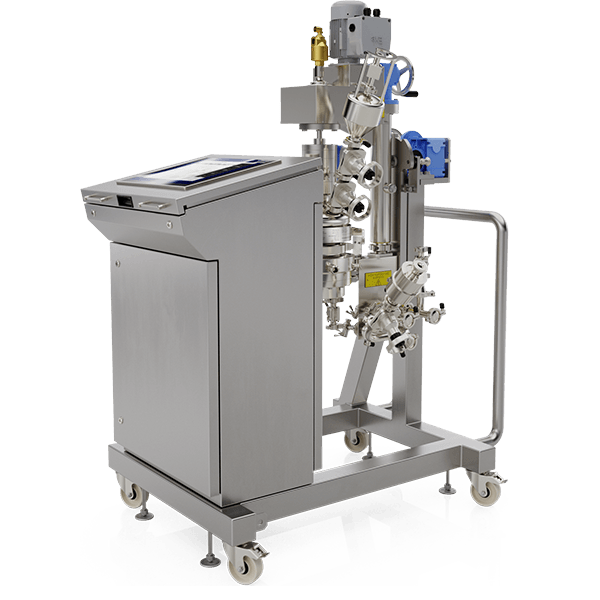
Features and Benefits
An All-In-One Solution
The purpose of the LabMSR™ is to provide R&D staff with an innovative tool enabling them to complete reliable product scale-up activities and refine their process parameters while being in a laboratory environment. The LabMSR™ will allow its end-users to reproduce the following processes, in particular:
Putting Your Scale-Up On Auto-Pilot
Developing complex drug formulations such as microsphere delivery systems has traditionally been challenging for most drug manufacturers, big or small. Ensuring that the chosen process technology used at R&D or pilot stage can be fully scalable to larger production batches is crucial. Finding out too late that a microsphere process is not scalable can have devastating impact and cost millions of dollars to drug manufacturers.
The LabMSR™ is the ideal solution to bridge the gap between your early R&D development activities and your GMP clinical batch production. This unique technology was developed by PSL to put your scale-up development on autopilot and to ensure that your key process objectives (such as product yield, product quality and integrity, batch consistency, etc.) and key production parameters (such as filtration time, washing time, drying time, etc.) can be maintained at every scale.
Product Recovery – A Crucial Requirement
Microspheres are extremely valuable medicines to develop and produce. Every drug manufacturer has the imperative to maximise their product recovery in order to secure their competitive position in the global market. The core features of our LabMSR™ system were designed keeping this crucial requirement in mind.
As per our MSR™ MicroSphere Refiner for GMP production, the product recovery method used by our LabMSR™ is completed by tilting the vessel. Being an all-in-one solution, the LabMSR™ method also minimises the number of transfers and connections, hence naturally maximising product yield.
Production Agility, Delivered
The LabMSR™ is suitable for multi-product applications and is the ideal tool to develop a wide range of microsphere drugs. It was designed to allow manufacturers to develop their drug pipeline by remaining agile and adaptable to ever-changing production requirements.
| FILTRATION AGILITY | DRYING AGILITY | CLEANING AGILITY |
|---|---|---|
| Multi-zone filtration | Multi-zone drying | CIP spray-ball |
| Loop or single-pass filtration | Agitated vacuum drying (positive temp.) | Fully drainable design |
| Quick filtration media change-over | Freeze-drying | No product traps |
Your Process, PAT-Verified
The implementation of the Process Analytical Technology (PAT) initiative in the pharmaceutical industry has been encouraged by the US FDA in order to ensure better product quality control and quicker time to market.
The LabMSR™ technology has embraced the PAT initiative as it allows drug manufacturers to complete real-time monitoring of their microsphere product quality and integrity during various production stages such as filtration and drying processes (including lyophilisation). The integration of PAT reduces the need for trial and error and helps drug companies towards right-first-time manufacturing, hence reducing drug development times for microspheres – traditionally more tedious than regular drugs.